
Dirección Xeral de Saúde Pública Galicia
Early Diagnosis of HIV Infection in Health-Care Settings
Abstract: AbstractThe late diagnosis of HIV infection is a
major obstacle to meeting the goals set out with the 90-90-90
Strategy, hence the importance of promoting early diagnosis of HIV
using HIV diagnostic testing. This test may be a screening test or a
confirmatory test. The basic principles of testing are three:
counselling, informed consent, and confidentiality. The recommended
populations for conducting this test are, on the one hand, individuals
with suggestive symptoms (both with conditions indicating HIV
infection and with AIDS-defining illnesses) and, on the other hand,
individuals without suggestive symptoms (both routine tests as well as
targeted and mandatory tests)
Keywords: Early Diagnosis; HIV Testing
INTRODUCTION
Late diagnosis is one of the main obstacles to fulfilling the
UNAIDS 90-90-90 Strategy. Late diagnosis may be defined as the
diagnosis of individuals who have a CD4 cell count below 350
cells/µL at the time of diagnosis or who have an AIDS-defining
illness (regardless of the CD4 cell count).
Delayed diagnosis of HIV has important negative consequences both
individually and collectively: at the individual level, delayed
diagnosis increases morbidity and mortality, and at the collective
level, it increases the transmission of the epidemic throughout the
population and increases the costs of the social and healthcare
system.
In 2017, diagnostic delays in the European Union accounted for
49% of cases (CD4 > 350 cells/µl), which included 28% of cases
with advanced disease (CD4 > 200 cells/µl). In Spain, diagnostic
delays accounted for 48%, whereas in Portugal they accounted for 51%
(European Centre for Disease Prevention and Control, 2018).
Therefore, it is essential to promote early HIV diagnosis using HIV
testing
(World Health Organization, 2010).
TYPES OF HIV TESTS
The diagnosis of HIV infection is based on a two-step strategy.
First, a screening analysis is performed, which is followed by a
confirmatory analysis
(Ministerio de Sanidad Servicios Sociales e Igualdad y Plan Nacional sobre Sida, 2014).
1. Screening techniques
In healthcare settings, the technique of choice is the
fourth-generation Enzyme-Linked ImmunoSorbent Assay (ELISA), which
involves the simultaneous determination of the presence of
anti-HIV-1 and anti-HIV-2 antibodies, and the HIV-1 p24 antigen.
This technique has an advantage compared to the third generation,
which is that it reduces the window period between the acquisition
of the infection and the detection of a HIV-positive result to
have a duration of only 2 to 4 weeks
There are also rapid tests, usually conducted in community
settings, which will be discussed in the next article.
Both techniques are highly sensitive. Therefore, in the case of
a negative result, infection may be excluded, except in the case
of a recent infection (6 weeks for fourth-generation ELISAs and 3
months for rapid tests). If results are positive, further
confirmation is required
(Ministerio de Sanidad Servicios Sociales e Igualdad y Plan Nacional sobre Sida, 2014).
2. Confirmatory techniques
2. Confirmatory techniques
The most frequently used techniques are the Western Blot (WB)
and the third-generation Recombinant ImmunoBlot Assay (RIBA). They
have a high specificity and facilitate the detection of specific
antibodies against the different proteins of the virus.
Fourth-generation tests, which include direct detection of
virus components (p24 antigen or viral genome), are recommended
for children under 18 months of age
(Panel de expertos de Gesida y Plan Nacional sobre el Sida, 2019)
or for adults with inconclusive
results..
BASIC PRINCIPLES AND SCOPE OF THE TEST
The basic principles of HIV testing (“the three Cs”)
(European Centre for Disease Prevention and Control (ECDC), 2016)
are the following:
-
Counselling: the individual being tested will
receive brief pre-test information. In addition, individuals who
test positive will be guaranteed post-test counselling, referral
to the appropriate care services, and access to the type of ART
required.
-
Informed Consent: the informed consent of the
individual being tested is required (at least verbally) and must
be voluntary (except for the cases listed in section 2.3).
-
Confidentiality must be maintained for the test
results and for the fact of having requested it. The test must
also be accessible to the entire population and be available
free of charge.
Regarding the scope of application, it is recommended that the
test be performed in all healthcare centres, both in primary care
and in specialised care centres, as well as in sexually transmitted
infection (STI) clinics; with an emphasis on specialised services
with a lesser tradition in offering this test, such as dentistry,
gynaecology, haematology, gastroenterology, dermatology,
pulmonology, and neurology, as well as in the emergency
department.
RECOMMENDATIONS FOR TESTING
There are recommendations for testing individuals with clinical
suspicion of HIV infection, as well as asymptomatic individuals,
whether or not they report being involved in HIV risk practices.
When there is no clinical suspicion, a distinction is made between
routine, targeted, and mandatory offers for testing.
(Figure 1.
Algorithm of HIV test recommendations ).
(Table 1. Figure 1.
Algorithm of HIV test recommendations translation)
Figure 1. Algorithm of HIV test recommendations
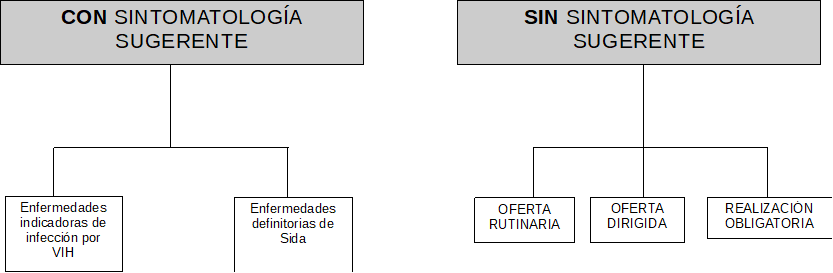
Source: Adapted from Spanish Ministry of Health, Social
Services and Equality (2014). Guía de Recomendaciones para el
diagnóstico Precoz del VIH en el ámbito sanitario.
Table 1. Figure 1.
Algorithm of HIV test recommendations translation)
|
Spanish
|
English
|
| CON SINTOMATOLOGÍA SUGERENTE |
WITH SYMPTOMS SUGGESTIVE OF HIV INFECTION |
| SIN SINTOMATOLOGÍA SUGERENTE |
WITH NO SYMPTOMS SUGGESTIVE OF HIV INFECTION |
| Enfermedades indicadoras de infección por VIH |
Illnesses indicating HIV infection |
| Enfermedades definidoras de Sida |
AIDS-defining illnesses |
| OFERTA RUTINARIA |
ROUTINE OFFER |
| OFERTA DIRIGIDA |
TARGETED OFFER |
| REALIZACIÓN OBLIGATORIA |
MANDATORY OFFER |
1. Individuals with clinical symptoms consistent with HIV
infection or AIDS
HIV testing is required for individuals with any of the
conditions listed in
(Table 2. AIDS-defining illnesses)
(Table 3. Conditions indicating HIV infection associated with an undiagnosed HIV prevalence of > 0.1 %)
(Table 4. Other conditions possibly associated with an undiagnosed HIV prevalence of > 0.1%)
(Table 5. Conditions in which the failure to identify the presence of HIV
infection may have significant negative consequences for the
clinical management of the individual despite the fact that the
estimated HIV prevalence is probably less than 0.1%).
Table 2. AIDS-defining illnesses
|
Doenças definidoras de
SIDA
|
| 1. Cervical cancer (invasive) |
| 2. Oesophageal candidiasis |
| 3. Pulmonary, tracheal, or bronchial candidiasis |
| 4. Coccidioidomycosis (disseminated or
extrapulmonary) |
| 5. Cryptococcosis (extrapulmonary) |
| 6. Chronic intestinal cryptosporidiosis (> 1 month
duration) |
| 7. HIV-associated encephalopathy |
| 8. Cytomegalovirus disease not affecting the liver,
spleen, and nodules |
| 9. Herpes simplex: chronic ulcers (>1 month
duration); or bronchitis, pneumonitis, or esophagitis |
| 10. Recurrent salmonella septicaemia |
| 11. Histoplasmosis (disseminated or extrapulmonary) |
| 12. Isosporiasis (chronic intestinal > 1 month
duration) |
| 13. Progressive multifocal leukoencephalopathy |
| 14. Immunoblastic lymphoma |
| 15. Primary cerebral lymphoma |
| 16. Burkitt lymphoma |
| 17. Mycobacterium avium complex or Mycobacterium
kansasii (disseminated or extrapulmonary) |
| 18. Mycobacterium, other species or unidentified species
(disseminated or extrapulmonary) |
| 19. Pneumonia (recurrent) |
| 20. Pneumocystis jirovecii pneumonia |
| 21. Cytomegalovirus retinitis (with loss of vision) |
| 22. Kaposi’s sarcoma |
| 23. HIV wasting syndrome |
| 24. Cerebral toxoplasmosis |
| 25. Mycobacterium tuberculosis (extrapulmonary or
pulmonary) |
| 26. Visceral leishmaniasis (kala-azar)* |
Table 3.Conditions indicating HIV infection associated with an
undiagnosed HIV prevalence of > 0.1 %
|
Conditions indicating HIV infection
associated with an undiagnosed HIV prevalence of > 0.1
%
|
| 1. Sexually transmitted infections |
| 2. Malignant lymphoma |
| 3. Anal cancer/dysplasia |
| 4. Cervical dysplasia |
| 5. Herpes zoster |
| 6. Hepatitis B or C (acute or chronic) |
| 7. Mononucleosis syndrome |
| 8. Thrombocytopenia or idiopathic leukocytopenia lasting
more than 4 weeks |
| 9. Seborrheic dermatitis/exanthema |
| 10. Invasive pneumococcal disease |
| 11. Fever with no apparent cause |
| 12. Candidaemia |
| 13. Visceral leishmaniasis |
Table 4. Other conditions possibly associated with an
undiagnosed HIV prevalence of > 0.1%
|
Other conditions possibly associated with
an undiagnosed HIV prevalence of >
0.1%
|
| 1. Primary lung cancer |
| 2. Lymphocytic meningitis |
| 3. Oral hairy leukoplakia |
| 4. Severe or atypical psoriasis |
| 5. Guillain-Barré syndrome |
| 6. Mononeuritis |
| 7. Subcortical dementia |
| 8. Multiple sclerosis-type disease |
| 9. Peripheral neuropathy |
| 10. Unexplained weight loss |
| 11. Idiopathic lymphadenopathy |
| 12. Idiopathic oral candidiasis |
| 13. Chronic idiopathic diarrhoea |
| 14. Idiopathic chronic kidney failure |
| 15. Idiopathic oral candidiasis |
| 16. Community-acquired pneumonia |
| 17. Candidiasis |
Table 5. Conditions in which the failure to identify the
presence of HIV infection may have significant negative
consequences for the clinical management of the individual despite
the fact that the estimated HIV prevalence is probably less than
0.1%
|
Conditions in which the failure to
identify the presence of HIV infection may have significant
negative consequences for the clinical management of the
individual despite the fact that the estimated HIV
prevalence is probably less than 0.1%
|
|
1. Conditions requiring aggressive immunosuppressive
treatment:
|
| 2. Primary space-occupying brain lesion |
| 3. Idiopathic thrombocytopenic purpura |
2. Individuals with no suspected HIV infection
In the case of individuals with no suspicion of infection, a
distinction must be made between routine, targeted, and mandatory
offers of HIV testing.
2.1 Routine offer of HIV testing
Routine offer of the test is a viable option considering its
costs and its degree of acceptability, as long as the conditions
detailed in Table 6 are met
(Table 6. Conditions for routine
offer of an HIV test):
Table 6. Conditions for routine
offer of an HIV test
|
Conditions for routine offer of an HIV
test
|
|
| General population (2 simultaneous criteria) |
|
| Pregnant women, preferably in the first trimester of
pregnancy |
|
| Prisoners in penitentiaries |
|
2.2 Targeted offer of HIV testing
Testing is offered to all individuals who, due to their
exposure to HIV or their background, need to rule out HIV
infection
(Table 7. Conditions for the targeted offer of HIV
testing)
(Table 8. Countries with an HIV prevalence of > 1%
in adults aged between 15 and 49 years old according to the
UNAIDS global report. Data from 2011.)
Table 7. Conditions for the targeted offer of HIV testing
|
Conditions for the targeted offer of HIV
testing
|
| All individuals who request it because they suspect a
risk exposure |
| Sexual partners of HIV-infected individualsa* |
| Current and former injection drug users and their
sexual partners |
| Men who have sex with men (MSM) and their sexual
partners (men and women)a |
| Individuals who practice prostitution: women, men, and
transgender people, their sexual partners, and their
clientsa |
| Heterosexual individuals with more than one sexual
partner and/or engaging in risky practices in the last 12
months |
| Individuals who wish to stop using condoms with their
regular partners |
| Individuals who have been sexually assaulted |
| Individuals who have had an occupational or accidental
exposure to HIV |
| Individuals from countries with a high-prevalence of
HIV infection (> 1%) and their sexual partners |
Table 8. Countries with an HIV prevalence of > 1% in
adults aged between 15 and 49 years old according to the UNAIDS
global report. Data from 2011
|
Countries with an HIV prevalence of >
1% in adults aged between 15 and 49 years old according to
the UNAIDS global report. Data from
2011.
|
|
| Sub-Saharan Africa |
Angola, Benin, Botswana, Burkina Faso, Burundi,
Cameroon, Central African Republic, Chad, Congo, Ethiopia,
Equatorial Guinea, Gabon, Gambia, Ghana, Guinea,
Guinea-Bissau, Ivory Coast, Kenya, Lesotho, Malawi, Mali,
Mauritania, Mozambique, Namibia, Nigeria, Rwanda, Sierra
Leone, South Africa, South Sudan, Swaziland, Tanzania,
Togo, Uganda, United Republic of Tanzania, Zambia,
Zimbabwe |
| Central and Western Europe |
Estonia |
| South and Southeast Asia |
Thailand |
| Middle East and North Africa |
Djibouti |
| Caribbean |
Bahamas, Haiti, Jamaica, Trinidad and Tobago |
| Latin America |
Belize, Guyana |
2.3 Mandatory HIV testing
In certain cases, HIV testing is legally mandated, as shown
in
(Table 9. Conditions for mandatory HIV testing):
Table 9. Conditions for mandatory HIV testing
|
Conditions for mandatory HIV testing
|
| Blood donations |
| Organ transplant, graft, or implantation |
| Donor studies |
| Users of assisted reproductive techniques |
| Semen collection and reception |
Acknowledgment
This publication has been possible to the cooperation program Interreg VA España-Portugal POCTEP - RISCAR 2014-2020.
http://www.poctep.eu
RINSAD
The Journal of Childhood and Health
(Revista Infancia y Salud - RINSAD),
ISSN: 2695-2785, arises from the collaboration between the
administrations of Portugal, Galicia, Castilla y León, Extremadura,
and Andalusia, within the
Interreg
Spain-Portugal RISCAR
project, and aims to
disseminate scientific articles on children’s health, providing
researchers and professionals with a scientific base from which to
learn about the latest advances in their respective fields.
The RISCAR project is co-financed by the European Regional Development Fund (ERDF) through the Interreg Program V-A Spain-Portugal (POCTEP) 2014-2020, with a total budget of € 649.699.
RINSAD is the result of the
Interreg
Spain - Portugal RISCAR
project in collaboration
with the
University
of Cádiz
and the
Nursing
and Physiotherapy Department of the University of
Cádiz
, Cádiz, Spain.
The works published in this journal are licensed under a
Creative
Commons Attribution-NonCommercial-ShareAlike 4.0
International
license.
References
-
European Centre for Disease Prevention and Control, 2018European Centre for Disease Prevention and Control, HIV/aids surveillance in europe 2018 - 2017 data, European Centre for Disease Prevention and Control, https://www.ecdc.europa.eu/en/publications-data/hivaids-surveillance-europe-2018-2017-data.
-
World Health Organization, 2010)World Health Organization, Scaling up hiv testing and counselling in the who european region as an essential component of efforts to achieve universal access to hiv prevention, treatment, care and support, 2010.
-
Ministerio de Sanidad Servicios Sociales e Igualdad y Plan Nacional sobre Sida, 2014Ministerio de Sanidad Servicios Sociales e Igualdad y Plan Nacional sobre Sida, Guía de recomendaciones para el diagnóstico precoz del vih en el ámbito sanitario, 2014.
-
Panel de expertos de Gesida y Plan Nacional sobre el Sida, 2019Panel de expertos de Gesida y Plan Nacional sobre el Sida., Documento de consenso de gesida/plan nacional sobre el sida respecto al tratamiento antirretroviral en adultos infectados por el virus de la inmunodeficiencia humana (actualización enero 2019), 2019.
-
European Centre for Disease Prevention and Control, 2016European Centre for Disease Prevention and Control, (ECDC), HIV testing: Evaluation of the ecdc guidance on hiv testing: Increasing uptake and effectiveness in the european union, 2016.


 License Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International
License Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International