
Luis Torres Pérez, Mónica Rodríguez Bouza, Ana María Leal Valle, Jesús Bujalance Hoyos and Cipriano Viñas Vera
Critical Reading: Treatment of Vomiting
Abstract: AbstractThis paper critically reviews a scientific article based on the following question: Is ondansetron the reference drug to address acute gastroenteritis in paediatric emergencies? The article by Freedman has been selected (Freedman et al., 2006). The results indicate a deficit in the methodology used when comparing ondansetron with placebo.
Keywords: Ondansetron; Emergency Treatment; Placebo
APPROACH TO THE PROBLEM
Although oral rehydration therapy is recommended for children with mild to moderate dehydration, it remains underused. Doctors in the emergency department are more likely to choose the intravenous route during oral rehydration when vomiting is a major symptom. In one survey, 36% of paediatricians reported that vomiting was a contraindication to oral rehydration. Therefore, a safe and effective method of controlling vomiting is likely to increase the utilisation rate and success of oral rehydration. This speeds up the approach in the emergency department and reduces the pressure in these units by discharging the child more quickly and safely.
One of the most popular medications due to its powerful antiemetic effect is ondansetron. However, several studies questioned its prescription in paediatric patients, due to its potential to generate heart arrhythmias and cause diarrhoea, among others, which becomes a relative contraindication in cases where diarrhoea is the predominant symptom, coupled with vomiting. This situation is very common in the approach to acute gastroenteritis in both paediatric and adult emergency departments.
In view of the foregoing, is ondansetron the reference drug to address acute gastroenteritis in paediatric emergencies?
(Freedman, Adler, Seshadri, & Powell, 2006).
Table 1:
Table 1.
|
Problem (P) |
Intervention (I) |
Comparison (C) |
Outcome (O) |
| General approach |
Treatment of vomiting in children |
Administration of ondansetron |
Placebo |
Reduced presence and frequency of vomiting, improved oral intake, less need for intravenous fluid replacement. |
| Specific approach |
Treatment of vomiting in children between 6 months and 10 years of age with acute gastroenteritis (in emergency departments) |
Administration of oral ondansetron (orally dissolving tablets) |
Placebo (same format) |
|
LET’S ANSWER THE QUESTION
Based on that question, we searched for evidence in reference sources and found the article by Freedman
(Freedman et al., 2006)(Freedman et al., 2006), published in a very high-impact journal. This author has continued to publish studies in this line of research and has been included in several systematic reviews, including the systematic review of the AAP.
A quick reading of the article focusing on the abstract suggests some very promising conclusions that seem to suit our question: “In children with gastroenteritis and dehydration, a single dose of oral ondansetron reduces vomiting and facilitates oral rehydration and may thus be well suited for use in the emergency department.” However, the methods section states: “We enrolled 215 children 6 months through 10 years of age who were treated in a pediatric emergency department for gastroenteritis and dehydration. After being randomly assigned to treatment with orally disintegrating ondansetron tablets or placebo…”. Additionally, the results section indicates: “As compared with children who received placebo, children who received ondansetron were less likely to vomit (14 percent vs. 35 percent; relative risk, 0.40; 95 percent confidence interval, 0.26 to 0.61), vomited less often (mean number of episodes per child, 0.18 vs. 0.65; P <0.001), had greater oral intake (239 ml vs. 196 ml, P=0.001), and were less likely to be treated by intravenous rehydration (14 percent vs. 31 percent; relative risk, 0.46; 95 percent confidence interval, 0.26 to 0.79).”
There is therefore a clear difference in favour of this medication compared to placebo. Is this the answer we are looking for?
UNPRETENTIOUS CRITICAL READING
The “overall quality” of a clinical research study is a complex concept that includes three elements:
-
The first element is clinical relevance, i.e. questions and, above all, research outcomes that are useful for clinical decision-making.
-
The second element is “methodological quality” or the extent to which study design, realisation, and analysis minimise selection bias, measurement bias, and confusion bias, i.e., the extent to which the study is valid or, so to speak, the extent to which the results are credible.
-
The third element is the applicability or transferability of the results to a specific patient (or to a specific group of patients), considering the other elements influencing the application of that evidence (the “representativeness” of the patients in that specific RCT, risk/benefit balance, availability, patient values, costs, etc.).
One of the key aspects that has been pointed out as “uncertain” in the reading is COMPARISON. This is very much the practical and ethical domain of the RCT. From the practising clinician’s point of view, it only makes sense to compare new interventions with interventions with already proven effects, or at least with the usual treatments. This reproduces the real possible decision-making dilemma, i.e. new treatment versus usual treatment. Not using proven treatments in these comparisons would therefore be maleficent.
Comparative interventions reflect the point of friction between two different dialectic styles, i.e. that of clinical practice and that of clinical research. Comparative interventions are therefore a vital issue that determines the study design in a number of ways.
Firstly, comparative interventions require an explicit knowledge of the state of the art of the treatments available for the health condition or clinical setting in question (preferably by means of a systematic review). Secondly, the existence of effective treatments limits the use of placebo as a research technique and makes it necessary to include effective treatments in comparisons.
And here is the question: Are there other well-known and frequently used antiemetic treatments for the proposed scenario?
Comparisons with placebo inevitably lead to a selection bias regarding the asymmetric comparison between an effective drug and a passive element (or an element from which no outcomes are expected). In addition, this type of comparison may lead to an overestimation of the drug’s results in terms of the proposed objective, which is to be prescribed as an element that reduces the presence of an event (in this case, vomiting) in the chosen population and setting.
As a result, the correction of the model clashes with the relevance of the chosen comparison model.
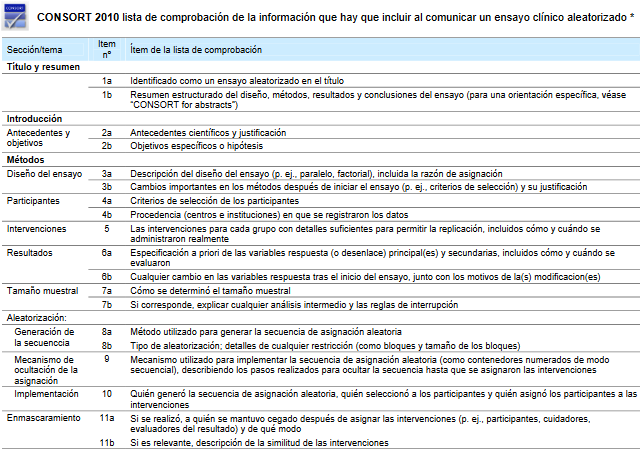
The most widespread review tool for clinical trials (CONSORT) does not point to evident shortcomings in the article.
See Image 1. Sample checklist of information to include when reporting a randomised clinical trial.
A FINAL THOUGHT
The Declaration of Helsinski specifies when placebo can be used in clinical research: “The benefits, risks, burdens and effectiveness of a new method should be tested against those of the best current prophylactic, diagnostic, and therapeutic methods. This does not exclude the use of placebo, or no treatment, in studies where no proven prophylactic, diagnostic or therapeutic method exists.”
Ondansetron may be a first-line drug in the clinical setting in question
(Higgins & Green, 2011),
but it is no less true that the comparison method chosen is quite unfortunate.
Choosing these comparison models is not uncommon, even in prestigious and international studies. There is an example where a bizarre title shows how this practice is prevalent in scientific studies. Using a correct methodology is not enough to ensure quality
(Tomasik et al., 2016)
See Image 2 Critical reading
Table 2. Critical reading Translation.
Table 2. Critical reading Translation.
| Spanish |
English |
| Lectura crítica |
Critical reading |
| Es una lectura activa, compleja y debidamente elaborada de
una obra escrita. |
Critical reading is the active, complex, and properly
conducted reading of a written work. |
| La lectura activa apela a la necesidad de comprensión
cabal del texto escrito. |
Active reading addresses the need for a thorough
understanding of a written text. |
| La lectura compleja aspira a ampliar el nivel de análisis
y comprensión de la información obtenida. |
Complex reading aims to broaden the level of analysis and
understanding of the information obtained. |
| La lectura debidamente elaborada consiste en relacionar lo
que se obtiene directamente del texto y aquello que se infiere
de él, con información extraída de otro texto. |
A well-conducted reading consists of connecting that which
is obtained directly from the text and that which is inferred
from it, with information extracted from another text. |
| Esta metodología aspira a desarrollar y fortalecer… |
This method aims to develop and strengthen... |
| La capacidad de razonamiento lógico y juicio crítico. |
The capacity for logical reasoning and critical
judgement. |
| La capacidad de identificar puntos de vista personales,
confrontándolos con los propios del texto. |
The ability to identify personal points of view and
compare them with those in the text. |
| La capacidad de identificar en el texto falacias de
razonamiento, sesgos, inconsistencias estructurales, faltas de
confiabilidad, etc. |
The ability to identify in the text logical fallacies,
biases, structural inconsistencies, lack of reliability,
etc. |
| “El que lee mucho y anda mucho, ve mucho y sabe mucho”.
Miguel de Cervantes Saavedra |
“He who reads a lot and walks a lot, sees a lot and knows
a lot.” Miguel de Cervantes Saavedra. |
See Image 3
Image 3. Article

It is also worth noting that, at the end of the selected article, in the section on conflicts of interest and acknowledgements, it is indicated that the study had been supported, among other institutions, by GlaxoSmithKline, the company that developed the molecule under the commercial name of Zofran®.
Thus, the use of ondansetron for these clinical features in question would require a review comparing the efficacy of ondansetron with other drugs, as there are numerous alternatives that have yielded positive results and are safe and widely used
(Meredith, Murray, & McMurray, 2004)
Ver Image 4
Image 4. Clipping

Acknowledgment
This publication has been possible to the cooperation program Interreg VA España-Portugal POCTEP - RISCAR 2014-2020.
http://www.poctep.eu
RINSAD
The Journal of Childhood and Health
(Revista Infancia y Salud - RINSAD),
ISSN: 2695-2785, arises from the collaboration between the
administrations of Portugal, Galicia, Castilla y León, Extremadura,
and Andalusia, within the
Interreg
Spain-Portugal RISCAR
project, and aims to
disseminate scientific articles on children’s health, providing
researchers and professionals with a scientific base from which to
learn about the latest advances in their respective fields.
The RISCAR project is co-financed by the European Regional Development Fund (ERDF) through the Interreg Program V-A Spain-Portugal (POCTEP) 2014-2020, with a total budget of € 649.699.
RINSAD is the result of the
Interreg
Spain - Portugal RISCAR
project in collaboration
with the
University
of Cádiz
and the
Nursing
and Physiotherapy Department of the University of
Cádiz
, Cádiz, Spain.
The works published in this journal are licensed under a
Creative
Commons Attribution-NonCommercial-ShareAlike 4.0
International
license.
References
-
Freedman, Adler, Seshadri, & Powell, 2006Oral Ondansetron for Gastroenteritis in a Pediatric Emergency Department. (2006, 20 04). pp. 1698-1705,
354
(16), doi:10.1056/nejmoa055119, FreedmanStephen B.AdlerMarkSeshadriRoopaPowellElizabeth C., New England Journal of Medicine.
-
Manual Cochrane de revisiones sistemáticas de intervenciones, 2011Higgins, J. P. T., & Green, S. (2011). Centro Cochrane Iberoamericano. Madrid: Cochrane Iberoamérica. Manual Cochrane de revisiones sistemáticas de intervenciones.
-
Meredith, Murray, & McMurray, 2004A putative placebo comparison of the SCOPE and LIFE trials. (2004, 06). pp. 59-63,
5
(2), doi:10.3317/jraas.2004.011, MeredithPeter AMurrayLilian SMcMurrayJohn JV, Journal of the Renin-Angiotensin-Aldosterone System.
-
Tomasik et al., 2016Systematic review with meta-analysis: ondansetron for vomiting in children with acute gastroenteritis. (2016, 11 07). pp. 438-446,
44
(5), doi:10.1111/apt.13728, TomasikE.ZiółkowskaE.KołodziejM.SzajewskaH., Alimentary Pharmacology & Therapeutics.



 License Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International
License Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International